What is energy?
Ability or capacity to do work
Potential Energy
Stored energy resulting from the relative position of matter rather than its motion
Kinetic Energy
A body or particle's energy due to its motion
Open & Closed Systems
- An open system exchanges energy with its surroundings
- A closed system does not receive or emit energy
- Thermodynamically, the Earth is an open system
- However, materially, it is a closed system
First Law of Thermodynamics
First Law of Thermodynamics
- Conservation of energy
- Energy can be transformed, but not destroyed (or created)
- Change in internal energy equals heat added to the system minus work done by the system
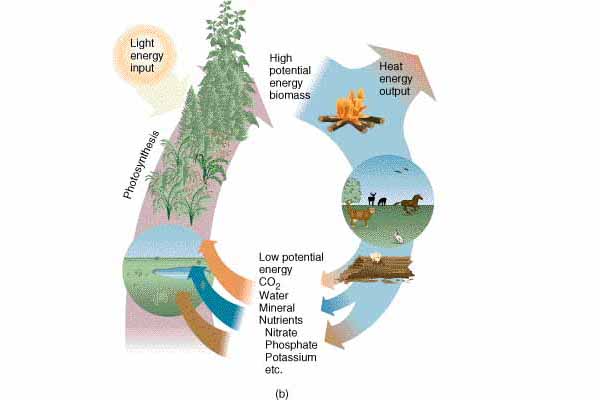
Second Law of Thermodynamics
Second Law of Thermodynamics
- Law of entropy
- With every transformation, some energy is lost to heat
- No conversion is 100% efficient
- Heat tends to flow from hot to cold regions and energy tends to flow from mechanical to heat energy
- Any flow against these trends must be compensated by an offset in the opposite direction (Gamow, 2006)
Subsections
Brian M Napoletano
2011-09-12